Stem Cell Trial Helps Paralyzed Man Walk Again—Americans Could Wait a Decade or More for Similar Treatment
A Tokyo man once told he'd never walk again is now standing against all odds–thanks to a cutting-edge stem cell treatment. But in the U.S., patients with similar injuries could face decade-long delays, as breakthroughs like this navigate the slow, uncertain path to FDA approval and insurance coverage.
The new findings come from a clinical trial at Keio University, led by Dr. Hideyuki Okano, where four men with severe spinal cord injuries received injections of neural stem cells—engineered from reprogrammed adult cells. One participant can now stand and is relearning how to walk. Another regained partial movement in all four limbs. The other two showed no major change. The trial began in late 2021; researchers shared the latest results on March 21.
"That person is now training to walk," Okano said at a press conference. “This is a dramatic recovery.”
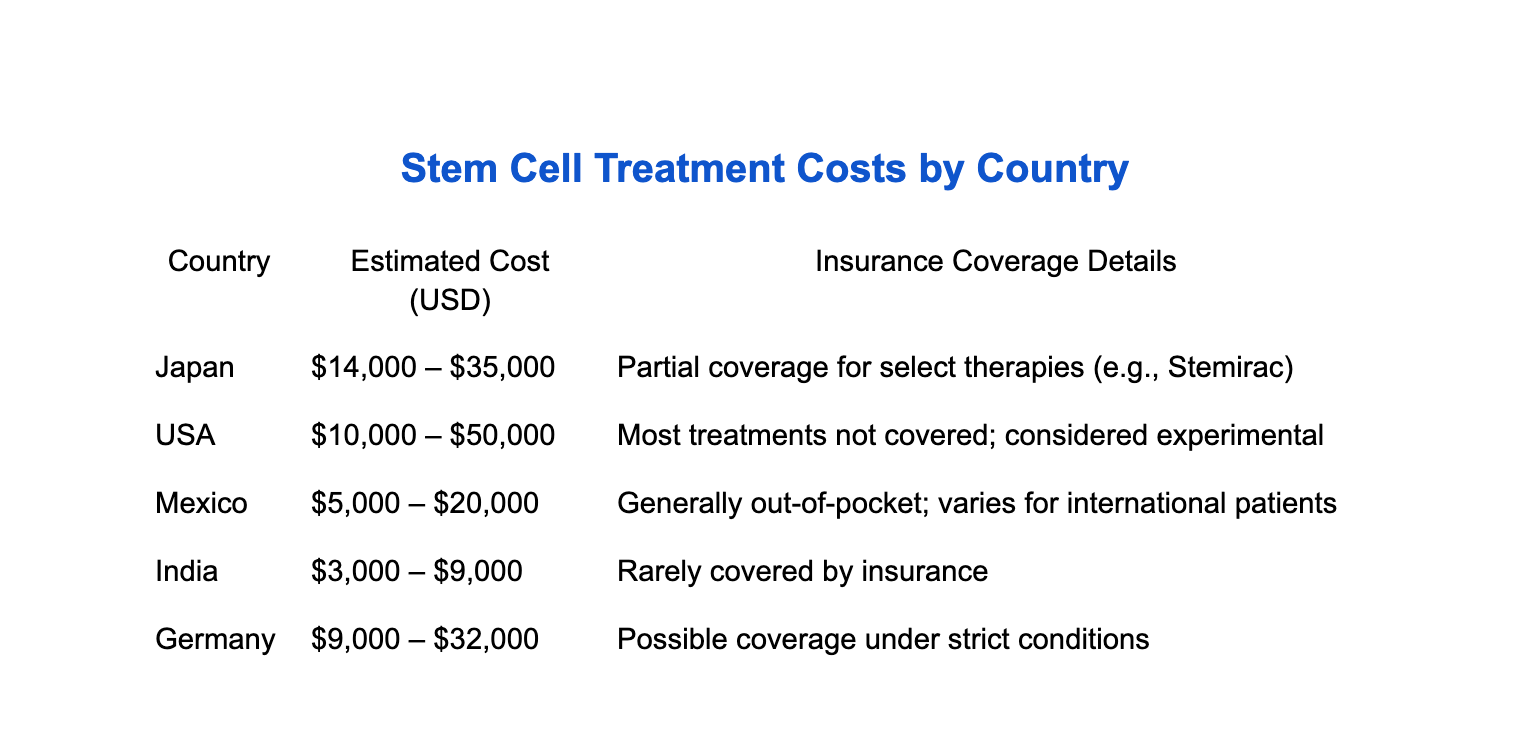
Japan has already greenlit—and funded—some regenerative treatments like Stemirac for spinal injuries. In contrast, most stem cell therapies in the U.S. are classified as experimental, limiting access to the hyper wealthy or those with military-level insurance.
In the U.S., a single stem cell treatment could cost patients anywhere from $10,000 to $50,000 out of pocket. Unlike Japan—where the government has stepped in to fund some therapies—American insurers largely refuse to cover stem cell interventions. Without FDA approval, they’re deemed investigational.
Dr. James St John, a neuroscientist at Australia’s Griffith University, said the results are “very exciting for the field,” but noted that natural recovery cannot be ruled out in individual cases without larger, controlled studies to confirm the treatment’s impact.
“Sometimes when people have spinal cord injury, they just naturally get recovery,” he added.
That caveat matters for U.S. regulators and insurers, who require rigorous, ironclad data before approving treatments for widespread use. For now, the results, while promising, are still in a kind of medical limbo.
It’s also important to note the risks of therapies that bypass FDA oversight. Some unapproved stem cell treatments have been linked to serious complications—including infections, permanent disabilities, and even deaths.
Yet the gap between access to lesser controversial treatments and medical innovations isn’t new. Just last year, a Utah family’s insurer denied coverage for a $24,000 bionic arm for their 9-year-old daughter, born without a hand. Despite detailed documentation from doctors, Select Health claimed it wasn’t medically necessary. The family turned to crowdfunding to afford the device.
A Hot Take Times investigation analyzed multiple different FDA regulatory approval timelines, clinical trial developments, and insurance coverage considerations and found that widespread insurance coverage for stem cell treatments for spinal cord injuries may be achieved within 7 to 15 years from now.
For now, the only people likely to access stem cell therapy stateside are those with deep pockets, veterans in elite military units, or patients willing to travel abroad.
Sources:
- “What is the Average Cost of Stem Cell Therapy in 2025? What to Know,” Alttreatment.io, September 25, 2024
- “Stem Cell Therapy Cost (2024 Update),” DVC Stem, November 26, 2024
- “The Cost of Stem Cell Therapy in 2025,” BioInformant, January 4, 2025
- “Is Stem Cell Therapy Covered By Insurance?” DVC Stem, October 24, 2024
- “Stem Cell Therapy in Japan – Cost, Laws & Treatment Options (2025),” Alttreatment.io, December 23, 2024
- “6 Cheapest Countries for Stem Cell Treatment,” PlacidWay, January 21, 2025
- “Best Countries for Stem Cell Treatment,” Dr. Swiss Health Screening, January 24, 2025
- “Best Countries for Stem Cell Therapy in the World,” Universal Medical Travel (date not provided)
- “Stem Cell Treatment for Spinal Cord Injury,” Beike Cell Therapy, December 5, 2024
- “Clinical Translation of Stem Cell Therapy for Spinal Cord Injury Still in the Works,” BMC Medicine, September 4, 2022
- “Stem Cell Therapy in Spinal Cord Injury,” PubMed Central (PMC)
- “Usefulness of the Act on Safety of Regenerative Medicine,” PubMed Central (PMC), May 1, 2024
- “Important Patient and Consumer Information About Regenerative Medicine Therapies,” FDA.gov
- “What stem cell-based therapies are currently available?” Harvard Stem Cell Institute
- “Harms Linked to Unapproved Stem Cell Interventions Highlight Need for Greater FDA Enforcement,” Pew Charitable Trusts, June 2021




